In Vitro Diagnostics Industry Overview
The global in vitro diagnostics market size is expected to reach USD 113.38 billion by 2030, according to a new report by Grand View Research, Inc. It is estimated to register a CAGR of 0.2% over the forecast period driven by the increasing geriatric population, COVID-19 pandemic, and technological advancements in diagnostics that are supporting its adoption. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be one of the high-impact rendering drivers. Technological advancements were further accelerated by the launch of COVID-19 IVD diagnostics and enhanced the adoption of instruments and consumables for technologies, such as PCR. Competitors in the market are increasingly adopting agreement and partnership strategies to maintain a constant flow of business for manufacturers & diagnostics for users.
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics market based on product, technology, application, end-use, test location, and region:
Based on the Product Insights, the market is segmented into Instruments, Reagents, Services.
- The reagents products segment led the global market in 2021 and accounted for the largest revenue share of more than 65.00%.
- The region is expected to retain its dominance growing at the second-fastest CAGR during the forecast period owing to the extensive R&D initiatives being undertaken by major market players for the development of novel reagents. The launch of kits that enable faster detection of cancer is allowing companies to focus on niche profitable areas in the IVD business.
- The growth in precision medicineis projected to enhance the overall demand for such novel reagents and consumables. Players are aligning their instrument launches in line with the increasing genetic test requirement globally.
Based on the Technology Insights, the market is segmented into Immunoassay, Hematology, Clinical Chemistry, Molecular Diagnostics, Coagulation, Microbiology, Others.
- The molecular diagnostics application segment held the highest market share of 37.95% in 2021 owing to the launch of novel products and continuous evolution in technology.
- This can be attributed to the continued impact of the COVID-19 pandemic on the market and the enhanced need of consumers in the market. For instance, flu and COVID-19 have similar symptoms, which made differentiation difficult.
- This unmet need was fulfilled by the launch of novel products that could detect Flu as well as SARS-CoV-2. Companies, such as Roche, have made SARS-CoV-2 & Flu A/B Rapid Antigen Test available in the market to fulfill the demand.
- Diagnosis is done by plating patient samples and selecting pure cultures from the same. Antibiotic susceptibility testing is considered one of the fastest-growing applications in microbiology testing. A rise in the prevalence of pathogenic diseases is expected to fuel the growth of the microbiology segment.
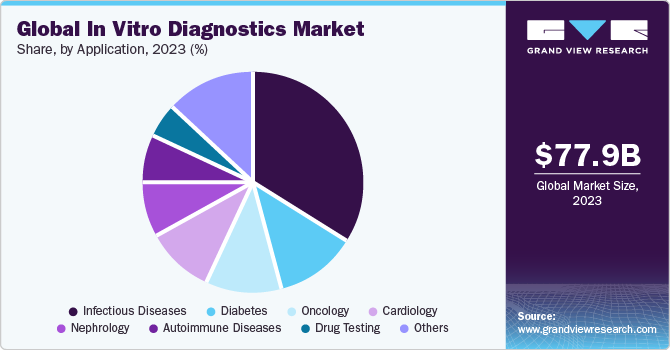
Based on the Application Insights, the market is segmented into Infectious Disease, Diabetes, Oncology, Cardiology, Nephrology, Autoimmune Disease, Drug Testing, Others.
- The infectious disease application segment held the highest market share of 59.49% in 2021. IVDs enable the detection of microorganisms that cause infectious diseases. The most common life-threatening infections are HIV/AIDS, tuberculosis, hepatitis, and pneumonia.
- The spread of COVID-19 has caused a sudden spike in uptake of IVD in this segment, leading to a higher market share.
- Diabetes is one of the leading causes of death globally. According to the International Diabetes Federation (IDF), in 2021, there were 537 million people across the globe suffering from diabetes, and the number is expected to increase to 643 million by 2030. Among the total number of people diagnosed with diabetes, about 49.7% remain undiagnosed.
- Cancer, a chronic disease, is one of the major causes of death globally. In February 2022, Cancer Moonshot was initiated by the U.S. government to enhance the screening rate for cancer for identifying the missed cases due to the COVID-19 pandemic.
Based on the End-use Insights, the market is segmented into Hospitals, Laboratories, Home Care, Others.
- The laboratory end-use segment led the global market in 2021 and accounted for the highest revenue share of more than 39.00%. An increase in awareness about personalized medicine, a rise in demand for affordable services, and technological advancements are some of the key factors expected to boost the growth of the laboratory segment.
- The demand for hospital-based IVD tests is increasing. Most of the IVD devices are purchased by hospitals and are used in significant volumes. In 2022, there are over 6,093 hospitals in the U.S. that require constant aid from IVD for critical decision-making, as IVD tests provide faster and more accurate results.
Based on the Test Location Insights, the market is segmented into Point of Care, Home Care, Others.
- The point-of-care test location segment held the largest revenue share of more than 20.5% in 2021. The demand for point-of-care tests and devices is rising owing to the increased demand for rapid identification of diseases in close proximity to patients to facilitate faster decision-making.
- The U.K. government granted funding of USD 20.12 million to the company to develop the system. Homecare testing location trend has witnessed a surge in recent years. These tests have been an important source to mitigate the spread of the SARS-CoV-2 pandemic.
In Vitro Diagnostics Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Key Companies Profile & Market Share Insights
Market players undertake this strategy to strengthen their product portfolios and offer diverse technologically advanced & innovative products to their customers. In January 2021, Abbott received FDA approval for its rapid handheld Traumatic Brain Injury (TBI) blood test. The first-of-its-kind test is used for assessing mild TBIs and concussions in patients.
Some prominent players in the global In vitro diagnostics market include
- Abbott
- bioMérieux SA
- Quidel Corp.
- Siemens Healthineers
- Bio-Rad Laboratories, Inc.
- Qiagen
- Sysmex Corp.
- Charles River Laboratories
- Quest Diagnostics
- Agilent Technologies, Inc.
- Danaher Corporation
- Becton Dickinson and Company
- Hoffmann-La Roche Ltd.
Order a free sample PDF of the In Vitro Diagnostics Market Intelligence Study, published by Grand View Research.
No comments:
Post a Comment